Ames company receives $1 million contract to advance Ebola vaccine
BioProtection Systems Corporation, a company located in Ames, was awarded a contract with the U.S. Defense Threat Reduction Agency to continue work on an Ebola vaccine.
August 6, 2014
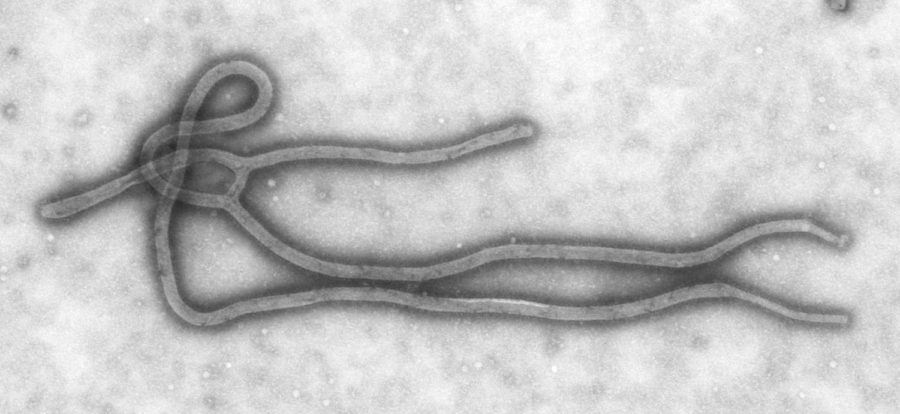
An Ames company has been awarded a contract to bring an Ebola vaccine closer to human testing. An Ebola outbreak in West Africa has killed almost 900 people and infected two American aid workers.
BioProtection Systems Corporation, a subsidiary of NewLink Genetics Corporation, was awarded the $1 million contract by the U.S. Defense Threat Reduction Agency.
“There is an urgent need for a medical countermeasure against the deadly Ebola virus,” said Dr. Charles Link, chairman and CEO of NewLink, in a news release.
The contract will fund preclinical toxicology studies and the manufacture of clinical materials. Additional funds may be given after final negotiation, according to the news release.
“This Ebola vaccine has been 100 percent effective in preventing lethal infection when given to nonhuman primates before they are infected with the virus,” Link said in the news release. “The vaccine also acts rapidly enough to have significant efficacy even when given to animals that have recently received a typically lethal dose of Ebola virus.”
Ebola is spread through direct contact with bodily fluids of infected people as well as indirect contact with surfaces contaminated by those fluids, according to the World Health Organization.
Ebola is commonly spread to health care workers “through close contact with patients when infection control precautions are not strictly practiced.”
Two American aid workers who were in Liberia to treat people infected with Ebola are being treated at Emory University in Atlanta, Ga., according to multiple media outlets.
“Advancing this vaccine into a human, Phase I safety study is a major priority for NewLink and our partners, whose ongoing support will be critical for moving the project forward,” said Dr. Nicholas Vahanian, president and chief medical officer of NewLink.