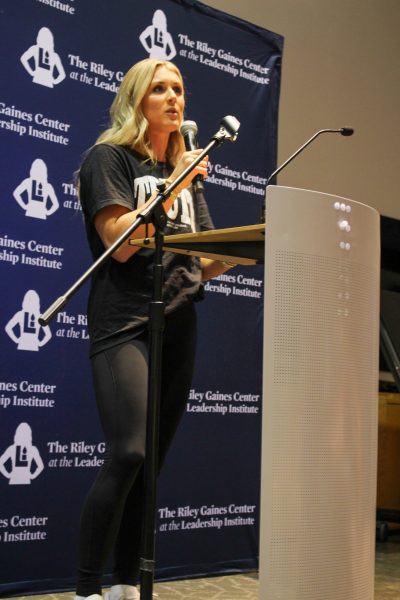Researchers add to Ebola study

Eshway Ramanan, right, and Mina Farahbakhsh use the handing drop vapor diffusion method as a way to set up crystals to get protein crystals. It’s one of many methods to get these results, but one that ISU students use most. Photo: Rebekka Brown/Iowa State Daily
January 27, 2010
ISU researchers contributed to efforts that reveal how the deadly Ebola virus functions inside the body.
“What we’re hoping is that the information we provided will help catalyze antiviral efforts,” said Gaya Amarasinghe, assistant professor of biochemistry, biophysics and molecular biology and leader of the ISU research team.
The Ebola virus is prevalent in equatorial regions of Africa.
The Zaire species of the virus, found to be the most deadly and most often studied, is what was used in this latest research.
No vaccine has been developed to treat the Ebola hemorrhagic fever.
The fever breaks out after a victim is infected with the virus, according to the World Health Organization.
The Ebola virus has the ability to replicate without triggering the host’s immune response.
Amarasinghe identified the structure of Ebola’s viral protein 35, or VP35, which contributes to its ability to circumvent detection.
“What was not known until recently was how the immune suppression worked,” he said.
Amarasinghe collaborated with Christopher Basler of Mt. Sinai School of Medicine in New York City, to discover how VP35 suppresses immune responses.
Mina Farahbakhsh, senior in biochemistry, and graduate student Parameshwaran Ramanan assisted with the research that has been published in Nature Structural and Molecular Biology.
The research team used non-infectious VP35 protein to identify Ebola’s protein-RNA structure. The next step was to identify how the protein functioned.
Cells in the body have evolved ways to protect against infection, Basler said. The Ebola virus is able to produce VP35, which binds to viral RNA and allows it to replicate without being detected by the host’s immune system.
“Ebola virus encodes VP35 protein to specifically block that immune response,” Basler said.
Identification of the structure and function of the Ebola virus VP35 protein may now lead to development of antivirals and additional treatments.
“We can better understand how this function contributes to the lethality of the virus,” Basler said.
If the protein’s function is critical for disease, scientists may be able to develop a drug to target and knock out that function.
VP35 is found in species of Ebola virus and Marburg virus. Both originated in Africa and are part of the Filovirus family.
However, the VP35 structural and functional information can allow researchers to compare and contrast it with other viruses.
The information provided by Amarasinghe and his ISU research team will enable other scientists to collaborate and investigate viral functions and potential anti-viral drug developments.
“The biggest thing I think for us was that we were able to do that with a combination of students and senior graduates,” Amarasinghe said.