Peterson: International engagement important on an individual level, too
Illustration: Dani Harris/Iowa State Daily
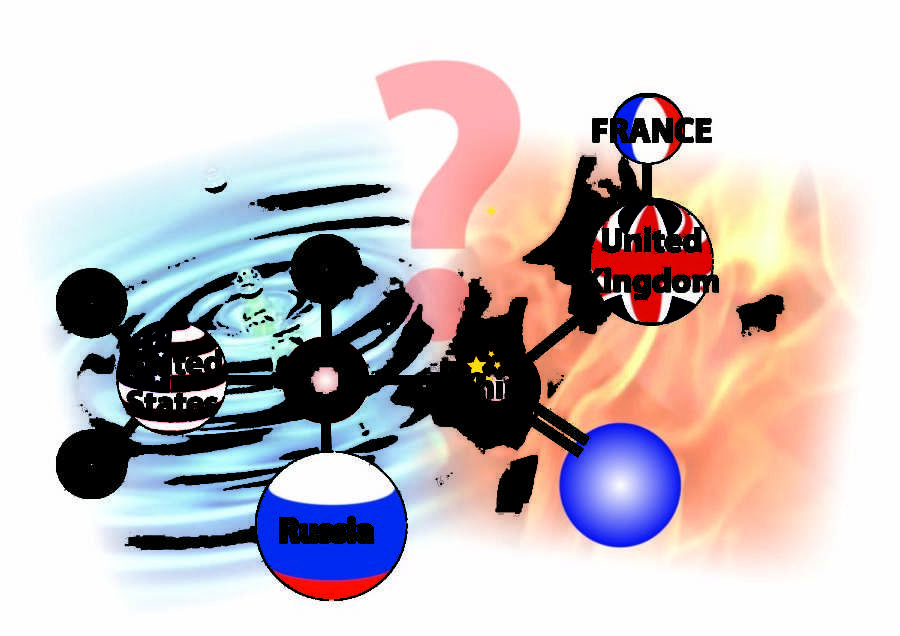
The relationships between global powers can easily be likened to the interactions of chemical compounds.
June 7, 2011
From within the political structure of Ames and Iowa State, the concepts of the United Nations and affairs in the Middle East or North Africa couldn’t seem more distant. That’s until the local gas stations are pumping gas at four dollars a gallon and the news fills up with humanitarian crises. But even at these points, we fail to understand how these situations came to be or the implications of our resolutions and their effects on the future.
International relations is often a daunting subject. With 192 countries in the world that constantly fluctuate, each with properties slightly more unique than the elements on the periodic table, the task of following international news can quickly become a professional activity. For those of us who don’t study the subject, we’re left unconcerned. What matters each day are simply the laws that regulate NASDAQ, Dow Jones, the S&P 500 and oil prices. But sometimes even those seem as foreign as the laws of quantum mechanics.
However complex international relations may be, and however distant it may seem, it’s important. Like the composition of chemical reactions, states can react with one another in various ways, among various groups, and in various environments. Carbon, hydrogen, oxygen and nitrogen are the building blocks for life and stability, and they might easily be compared with the five permanent members of the United Nations Security Council: China, France, Russia, the United Kingdom and the United States. Mixed correctly, these states help provide security and stability in the world. However, just as carbon, hydrogen, oxygen and nitrogen can be used to provide the compounds of life, so too can they be mixed to create an explosive compound called Trinitrotoluene (TNT).
It’s our responsibility as the ‘particles’ attracting and repelling one another, interacting to create our state, to understand how our actions will affect the international composition. By the same token, we must understand how the actions of other states will affect us. But unlike chemical reactions, we do not exist in a closed system. The United States and Russia might formulate an explosive reaction, but that reaction is by no means predetermined. Explosive reactions in international relations, unlike in chemistry, are not matters of course. By understanding the larger international environment, we can better protect ourselves from violent reactions and help sustain stability.
As individuals, there seems to be little we can do. But the United States is not atomized, like carbon. Within the composition, we constitute the forces that hold it together, and the signals we emit will determine the levels of attraction and repulsion between us and other states. We are the strong and weak forces of the state, and our actions hold sway over our relations with other countries.
Even though India is 8,000 miles from Iowa, and it’s 7,000 miles from here to Pakistan, the actions of these states are more important than they appear. What happens in international relations matters, even to those of us living in Ames. Maintaining stability between nations such as Palestine and Israel or India and Pakistan is critical to everyone living in this nuclear age. Perhaps more important is the fact that the behavior of all of us impacts the international scene.
States are constructions of their citizens, not atomized political units. This state of affairs gives us choice. We can choose to comply passively with the laws of physics like the electrons devoid of free will, or we can act to affect the international scheme and ensure stability and order. Unlike the elements, we do not necessarily have to make TNT or even any kind of destructive reaction. We can choose to support any kind of international reactions we want. All we have to do is get active in our own public and stay tuned into the world of international relations.