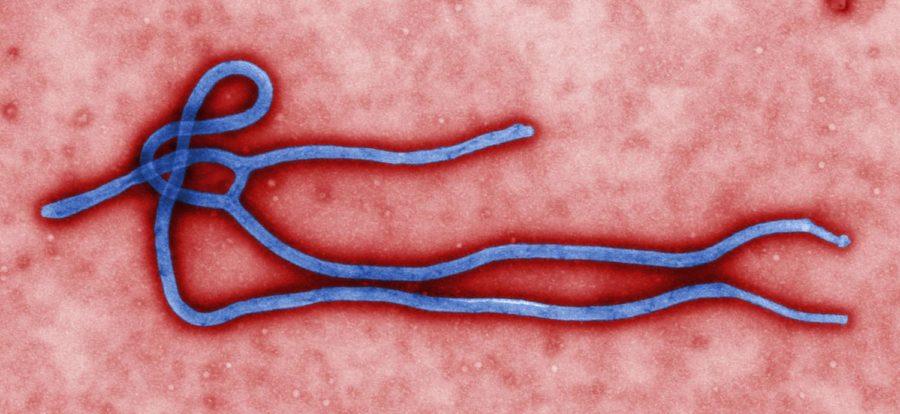
$50 million deal builds partnership for Ebola vaccine
NewLink Genetics, an Ames company housed in the Iowa State Research Park, has recently signed a $50 million deal with Merck & Co., Inc. to expedite the development of NewLink’s potential vaccine for the Ebola virus.
December 12, 2014
NewLink Genetics, an Ames company housed in the Iowa State Research Park, has recently signed a $50 million deal with Merck & Co., Inc. to expedite the development of NewLink’s potential vaccine for the Ebola virus.
NewLink received funding from the federal government in August to continue pre-existing research and development of its “rVSV-EBOV” vaccine candidate and has been working on it since.
Pam Eisele, a spokeswoman for Merck, said that the partnership went into effect Nov. 21 and that the $50 million deal was swift in nature. This was due in part to the recognition by NewLink that Merck was an ideal partner to help manufacture the vaccine.
The testing of vaccines is key in order to make sure they are safe and viable to the general public.
“Vaccines usually contain a part of a pathogen they’re trying to fight,” said Balaji Narasimhan, a professor in the chemical and biological engineering department.
Narasimhan, who has worked with NewLink on other projects, said that as of late, live parts of a pathogen are rarely used in vaccines. Instead, proteins that are specific to a virus are isolated and tested in vaccines.
“There are multiple levels of testing, including phases one, two, and three,” Narasimhan said.
NewLink is currently working on phase-one testing. This is the phase where vaccines are tested for safety in humans and not for effectiveness. As of Dec. 9, NewLink has garnered protection from any legal action taken against its development of the Ebola vaccine. While the declaration by the federal government applies only to the United States, other countries are being urged to follow suit in order to expedite the manufacture of a vaccine.
“We will see an accelerated time line,” Narasimhan said in reference to the development of the Ebola vaccine. This is due in part to the deadly nature of the disease and its recent and rapid emergence.
This accelerated time line has been somewhat stifled as of Dec. 11 due to reports of joint pain in the hands and feet of four patients in the phase-one trial of the vaccine in Geneva, Switzerland.