Editorial: Time for change regarding blood donations
April 1, 2021
According to the World Health Organization, human immunodeficiency virus (HIV) continues to be a major global public health issue, having claimed almost 33 million lives so far. It can affect people of all ages, races, genders and sexual orientations, but one group is often singled out as the “carrier” of it: gay men. This notion that gay men are the carriers of HIV/AIDS strains from the historical accusation of HIV/AIDS being the “gay disease,” as well as the systemic oppression of the queer community, which has ultimately led to men who have sex with men (MSM) being banned from donating blood for three months after they have sex.
The blood donation ban for MSM, a restriction that prevented any man who had sex with another man regardless of sexual orientation, from donating blood was originally much worse.
Originating in 1983 during the height of the HIV and AIDS epidemic in the United States, the policy was created as an emergency measure to prevent contamination of the U.S. blood supply by this deadly disease. The policy impacted MSM, women who have sex with MSM and transgender people that could be considered MSM.
The Food and Drug Administration (FDA) continued to enforce a lifetime ban on MSM from donating blood until December 2015, when they moved the ban to a deferral of one year for any MSM during the previous 12 months.
Then, the COVID-19 pandemic hit, and on April 2, 2020, the FDA announced it was updating its policy regarding blood donations from MSM by reducing the deferral period from 12 months to three months.
The FDA guidance “Revised Recommendations for Reducing the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products” states, “Defer for three months from the most recent sexual contact, a man who has had sex with another man during the past three months.” All U.S. blood collection organizations must follow this federal requirement.
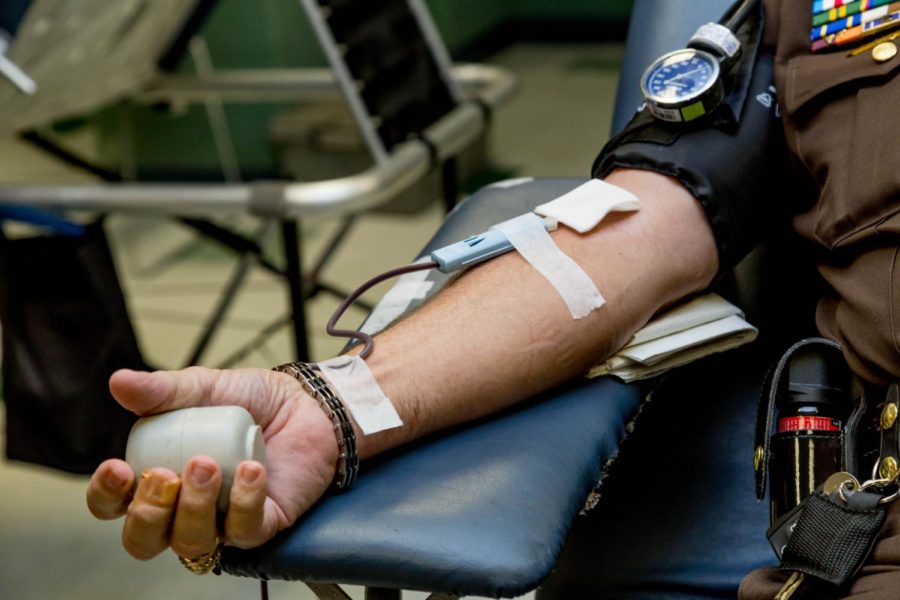
Whenever someone wishes to donate blood, there is a screening process. Blood centers nationwide screen potential donors by asking a set of questions written to determine risk factors that could indicate possible infection with a transmissible disease; that is when potential donors run into the questions about their sexual encounters with MSM.
The American Red Cross states on their website that “the gender-specific donation criteria and questions on the health history questionnaire are designed to ensure that the blood collection process is as safe as possible for the donors as well as for the recipients of blood.”
That statement and the policies set forth by the FDA have issues set within them that need to be addressed and changed. We, as the Iowa State Daily Editorial Board, are not saying there should be no questionnaire or no screening process, because the safety and quality of our blood supply is important. What we are saying is that parts of their policy and screening process need to be changed.
They need to be changed because the premise of the MSM blood donation deferral revolves around the assumption that MSM blood donors have a higher risk of contaminating blood supplies due to disproportionately high rates of HIV as compared to other groups in the U.S.
Though it is true that the MSM community has high rates of HIV as compared to other groups in the U.S., the current policy is wrong to make the assumption that all MSM could have HIV when not all MSM act the same. MSM vary widely in the number of sexual partners they have as well as in their engagement with risky sexual behaviors that determine HIV infection risks.
In reality, risk factors for HIV transmission, including behaviors such as having unprotected sex and having multiple sex partners, are identical, regardless of someone’s sexual orientation. The FDA’s blood donation deferral ignores these facts, treating MSM as a homogenous group with an identical risk for contracting HIV.
This means that under current guidelines, a monogamous gay man on pre-exposure prophylaxis (PrEP) who has had protected sex in the past three months with a long-term male partner would not be able to donate blood, while a heterosexual man who has had unprotected sex with multiple female partners in the same time period would be allowed to donate.
The Human Rights Campaign provides another example on their website: a man who has had protected oral sex with another man once in the three months is currently barred from donating blood. Yet, a woman who has had unprotected sex with multiple partners over the same time frame with no knowledge of their personal histories remains in the donor pool.
Situations like this show how blood donation deferral policies based on sexual orientation are rooted in prejudices as opposed to scientific evidence.
Another issue that comes up when you look at the deferral policy is that this policy is presented as a way to protect the greater public from HIV/AIDS by denying MSM the ability to donate blood if they have sex within the prior three months, but the fact is the Red Cross tests every unit of blood that is donated, regardless of donor.
Every donated unit of blood undergoes a rigorous series of tests to determine any possible presence of HIV, hepatitis, syphilis and other blood-borne disease. None of these tests, however, are 100 percent accurate, and they can produce faulty results.
For instance, despite current restrictions and testing of approximately 12 million units donated each year, 10 HIV-infected units have slipped through.
Yes, some HIV-infected units of blood passed through the testing process, but look at the numbers: 10 out of 12 million. That is such a small percentage and can be blamed on no one but the FDA for their testing abilities.
Getting rid of the ban on MSM donating blood is unlikely to raise that percentage as long as the FDA continues their rigorous testing abilities. Instead, we, as the Iowa State Daily Editorial Board, suggest putting in place a different policy that would better suit the current day and age.
We suggest screenings be based on sexual risk behavior rather than the gender of a prospective donor’s partner. “Risk behavior” could look like having multiple sexual partners in a short time period and having unprotected sex. This type of screening process is thus based on factors that could actually lead to a risk of HIV rather than homophobic leftovers from the ’80s.
And we are not the only ones who think this way: the American Public Health Association and the Human Rights Campaign have been suggesting similar changes for years.
Even the Red Cross supports moving away from the MSM policy, stating “The Red Cross recognizes the hurt this policy has caused to many in the LGBTQ+ community and believes blood donation eligibility should not be determined by methods that are based upon sexual orientation. We are committed to working toward achieving this goal.”
If these large national organizations can get behind the idea that the current FDA policies surrounding MSM are flawed, then it seems it is time the FDA starts making progress.