Engineers find “chameleon metals” through research
January 21, 2020
Iowa State engineers have found a way to change the surface structure in response to heat for liquid metal and potentially solid metal, according to a news release.
The changing of the metals is similar to how a chameleon changes its skin color in response to its environmental surroundings.
Martin Thuo, assistant professor of materials science and engineering, is the lead author of the paper “Chameleon Metals: Autonomous Nano-Texturing and Composition Inversion on Liquid Metals Surfaces.” The paper was first published in November 2019 and was then featured on the cover of the Angewandte Chemie journal on Jan. 2.
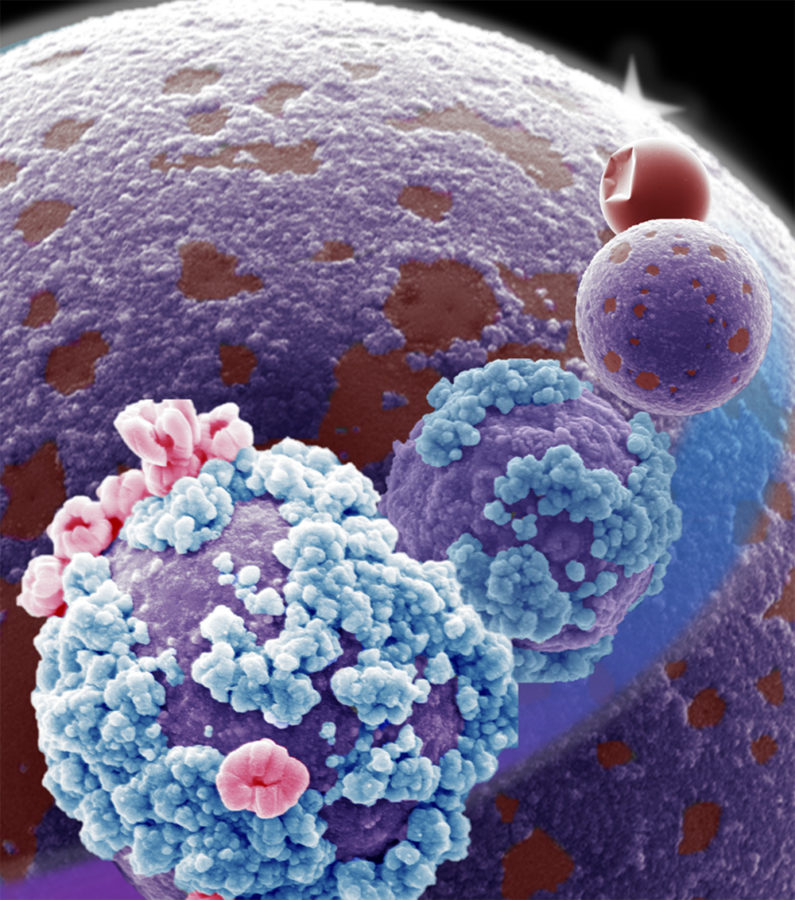
The paper reports that treating particles of liquid metal alloys with heat causes them to roughen their surfaces with tiny spheres or nanowires. Thuo said in the news release that if the heat is controlled, they can control surface patterns.
“The particles are responding to a certain level of heat and releasing a specific element based on temperature, just as a chameleon responds to the color of its environment,” Thuo said in the news release. “That’s why we say they’re chameleon metals – but responding to heat, not to color as the reptile does.”
Thuo and his research team wrote in their paper that this tunable surface patterning technology could “inspire design of ‘smart’ alloy systems that evolve the surface patterns and their composition with temperature—or analogous stimuli—for applications ranging from sensing to catalysis.”
The co-authors of the paper are: Andrew Martin, graduate materials science and engineering student, Winnie Kiarie, graduate electrical and computer engineering student and Boyce Chang, postdoctoral fellow at the University of California, Berkeley, who earned his doctoral degree at Iowa State.
The start of this research began with a liquid metal alloy of gallium, indium and tin synthesized into particles covered with a smooth oxide shell that has been chemically stabilized. As the research team heats the particles, the surface thickens, stiffens and starts to act more like a solid state of matter.
The surface breaks, which allows the liquid metal inside of it to come to the surface. The research found that the gallium breaks through first, being the most reactive. Indium comes to the surface with more heat, and at the highest heat of about 1,600 degrees Fahrenheit, tin comes through.
Kiarie said in the press release that the particles of the metal respond to a very controlled environment, where the levels of temperature and oxygen are controlled by the researchers.
In the paper the researchers wrote, it is stated that the movement from the layer under the surface allows the liquid metal particle to “continuously invert its composition under thermal stimuli.”
Having a controlled environment with controlled variables allowed the researchers to predict and program the precise surface texture of the particles.
This technology could be used to fine-tune the performance of a metal as a catalyst or its ability to absorb compounds, Martin said in the news release.
The technology will also work with other metal alloys.
“This is not unique to these materials,” Thuo said in the press release. “This is a behavior of metals in general. Other metals subject to the same treatment should do this. This is a universal property of metals.”
The full “Chameleon Metals: Autonomous Nano-Texturing and Composition Inversion on Liquid Metals Surfaces” paper can be read on the Wiley Online Library website, accessed by Iowa State.